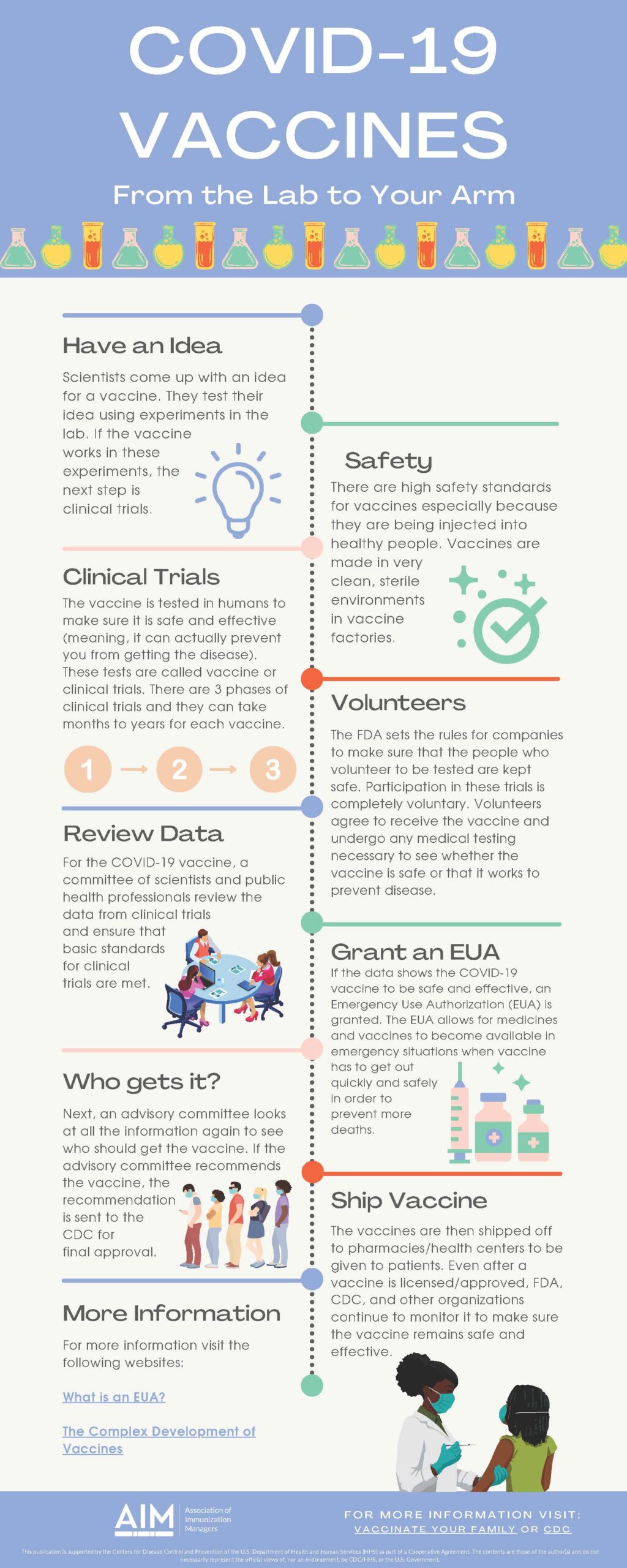
Vaccine Process
Have an Idea
Scientists come up with an idea for a vaccine. They test their idea using experiments in the lab. The next step is clinical trials to determine if the vaccine works in these experiments.
Safety
Vaccines have high safety standards, especially because they are being injected into healthy people. Vaccines are made in very clean, sterile environments in vaccine factories.
Clinical Trials
The vaccine is tested in humans to make sure it is safe and effective (meaning, it can actually prevent you from getting the disease). These tests are called vaccine or clinical trials. There are 3 phases of clinical trials, and they can take months to years for each vaccine.
Volunteers
The FDA sets the rules for companies to ensure that the people who volunteer to be tested are kept safe. Participation in these trials is completely voluntary. Volunteers agree to receive the vaccine and undergo any necessary medical testing to see whether it is safe or works to prevent disease.
Review Data
For the COVID-19 vaccine, a committee of scientists and public health professionals reviews the data from clinical trials and ensures that basic clinical trial standards are met.
Grant an EUA
If the data shows the COVID-19 vaccine to be safe and effective, an Emergency Use Authorization (EUA) is granted. The EUA allows for medicines and vaccines to become available in emergencies when vaccine has to get out quickly and safely to prevent more deaths.
Who Gets It?
Next, an advisory committee looks at all the information again to see who should get the vaccine. If the advisory committee recommends the vaccine, the recommendation is sent to the CDC for final approval.
Ship Vaccine
The vaccines are then shipped off to pharmacies/health centers to be given to patients. Even after a vaccine is licensed/approved, the FDA, CDC, and other organizations continue monitoring it to ensure it remains safe and effective.
More Information
For more information visit the following websites:
This publication is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) as part of a Cooperative Agreement. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.